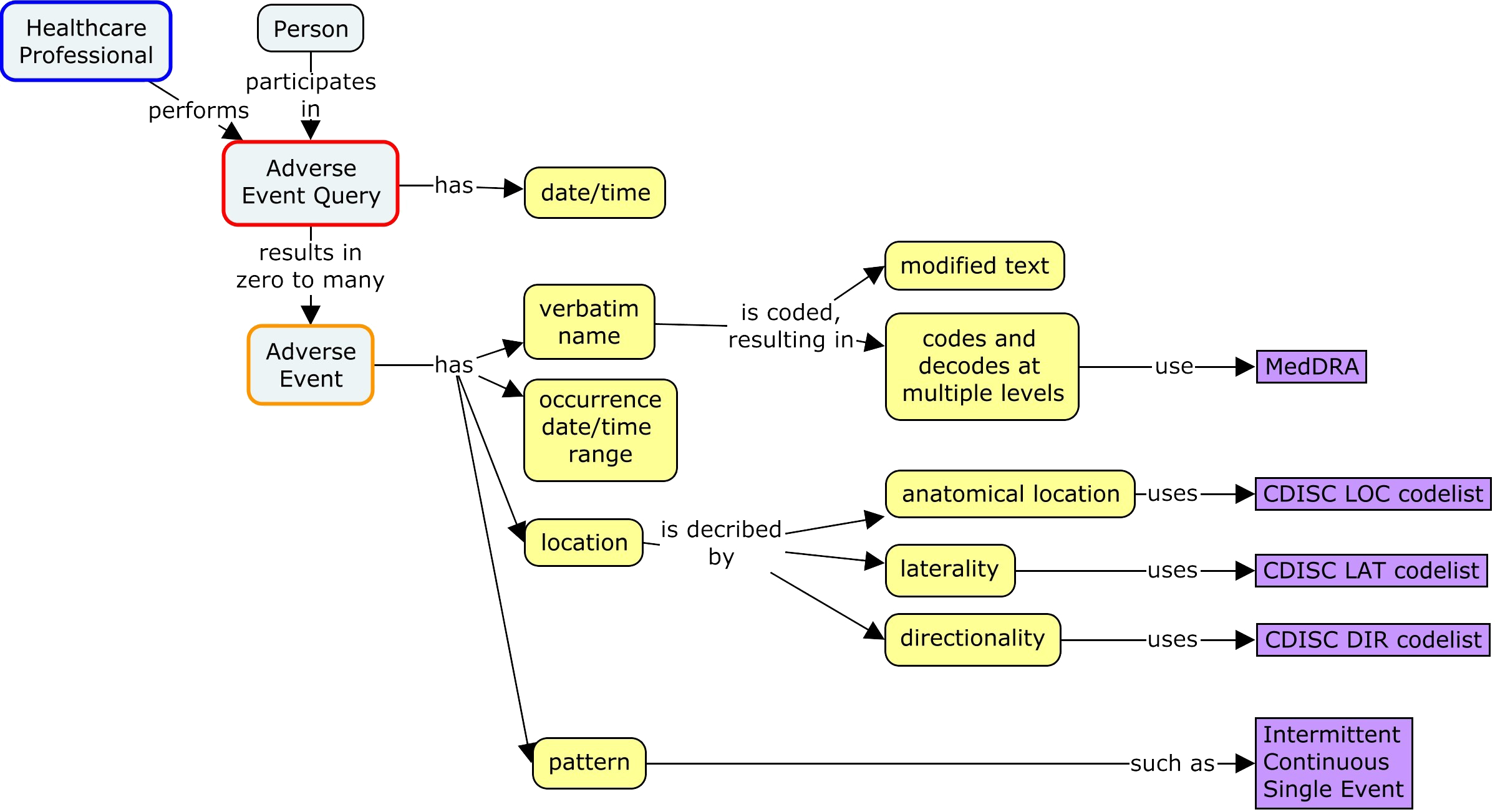
Controlled Terminology for Clinical Research: A Collaboration Between CDISC and NCI Enterprise Vocabulary Services

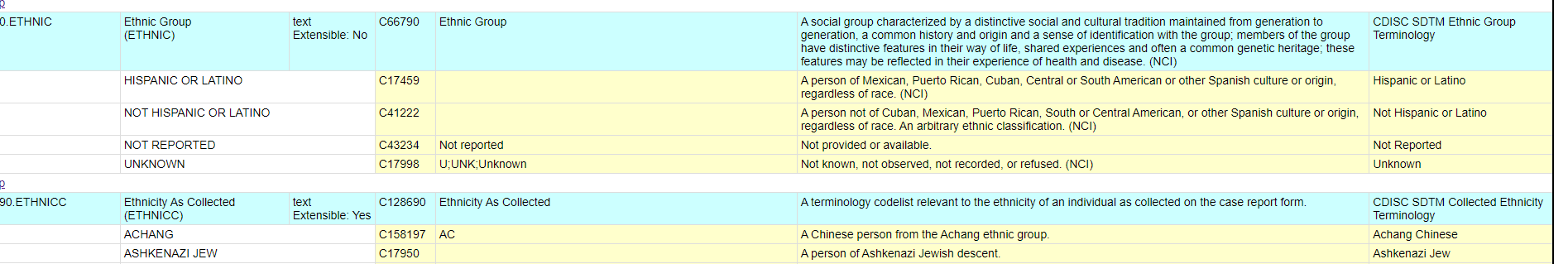
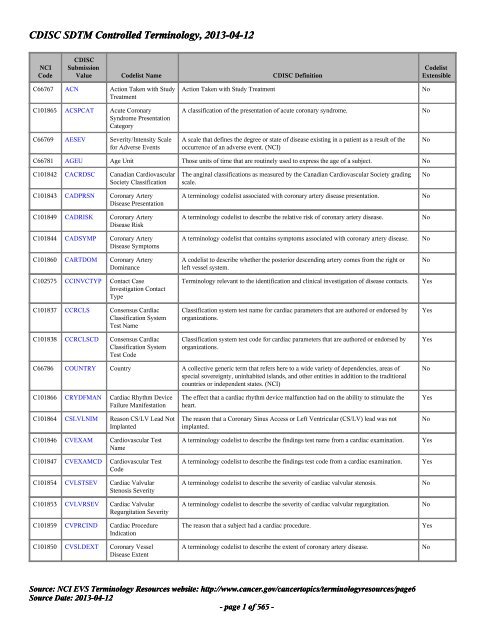
Figure 1 from From ACE to ZINC Examples on the use of SDTM Controlled Terminology for lab tests | Semantic Scholar
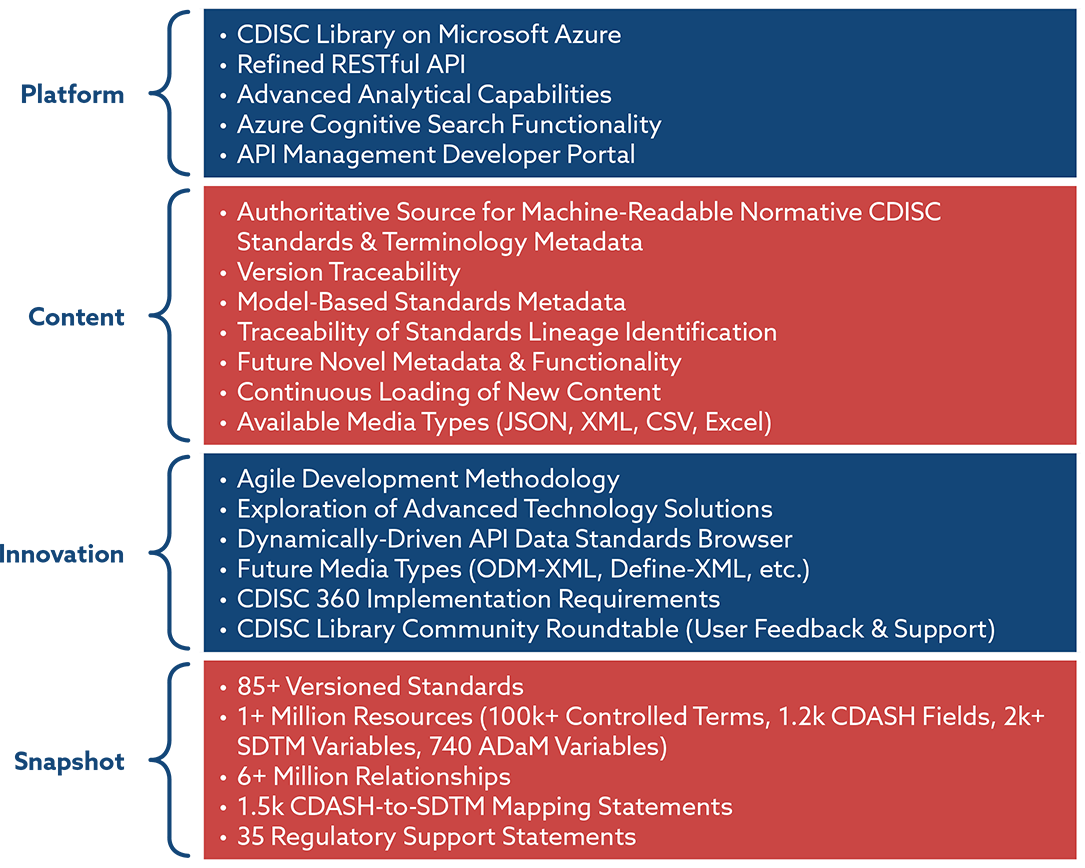
173-2012: Automatic Consistency Checking of Controlled Terminology and Value Level Metadata between ADaM Datasets and Define.xml

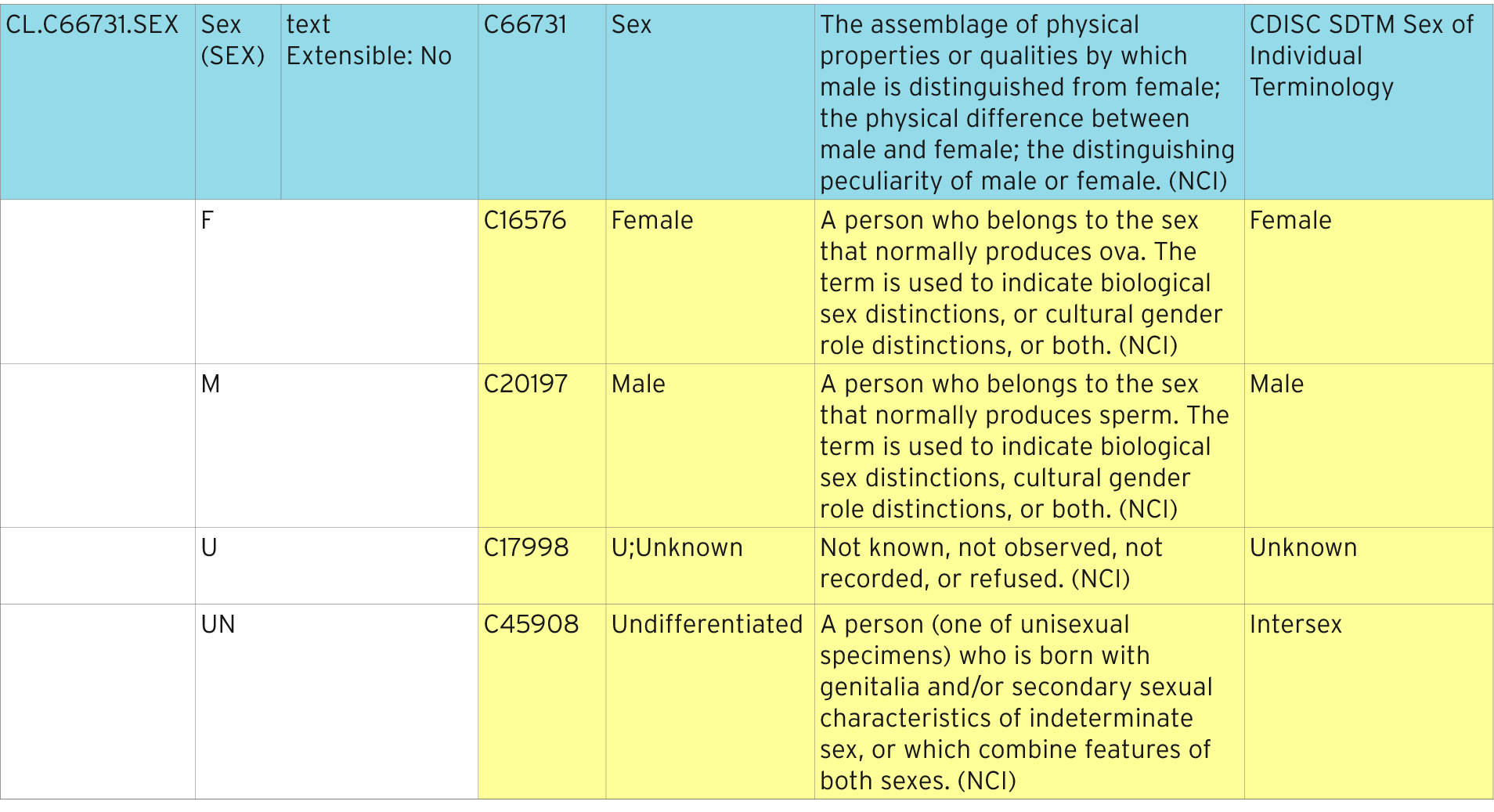
Table 4 from From ACE to ZINC Examples on the use of SDTM Controlled Terminology for lab tests | Semantic Scholar

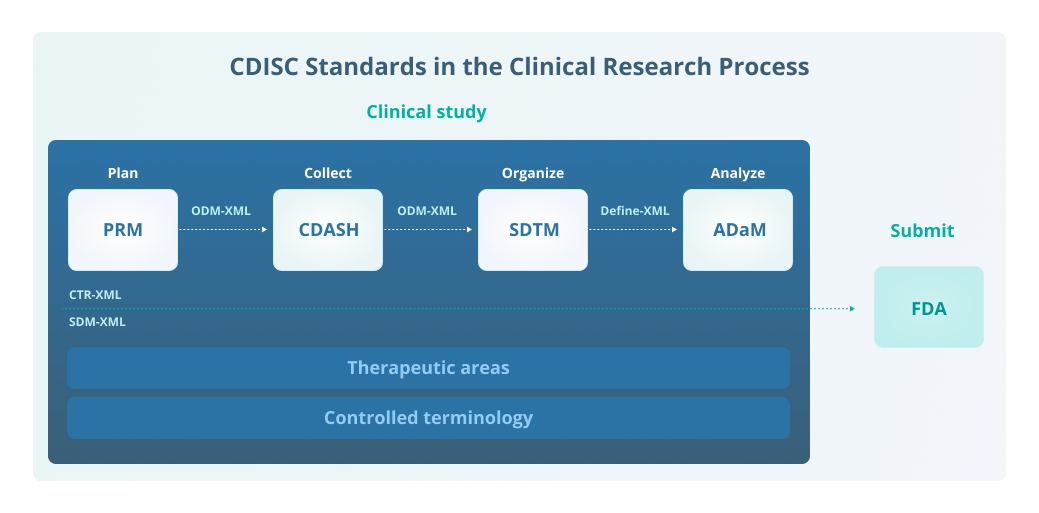
Original controlled terminology mapped to CDISC controlled terminology | Download Scientific Diagram