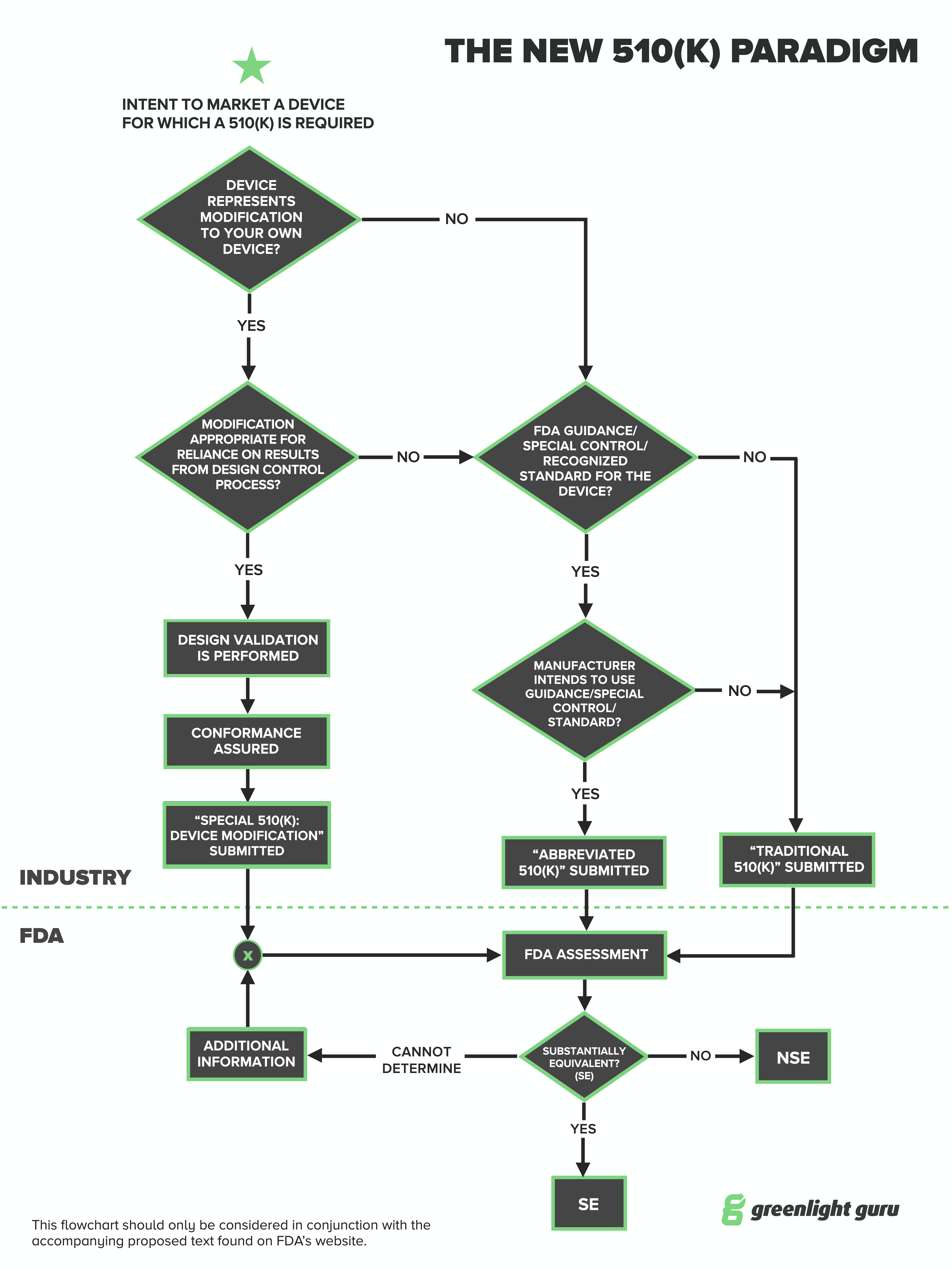
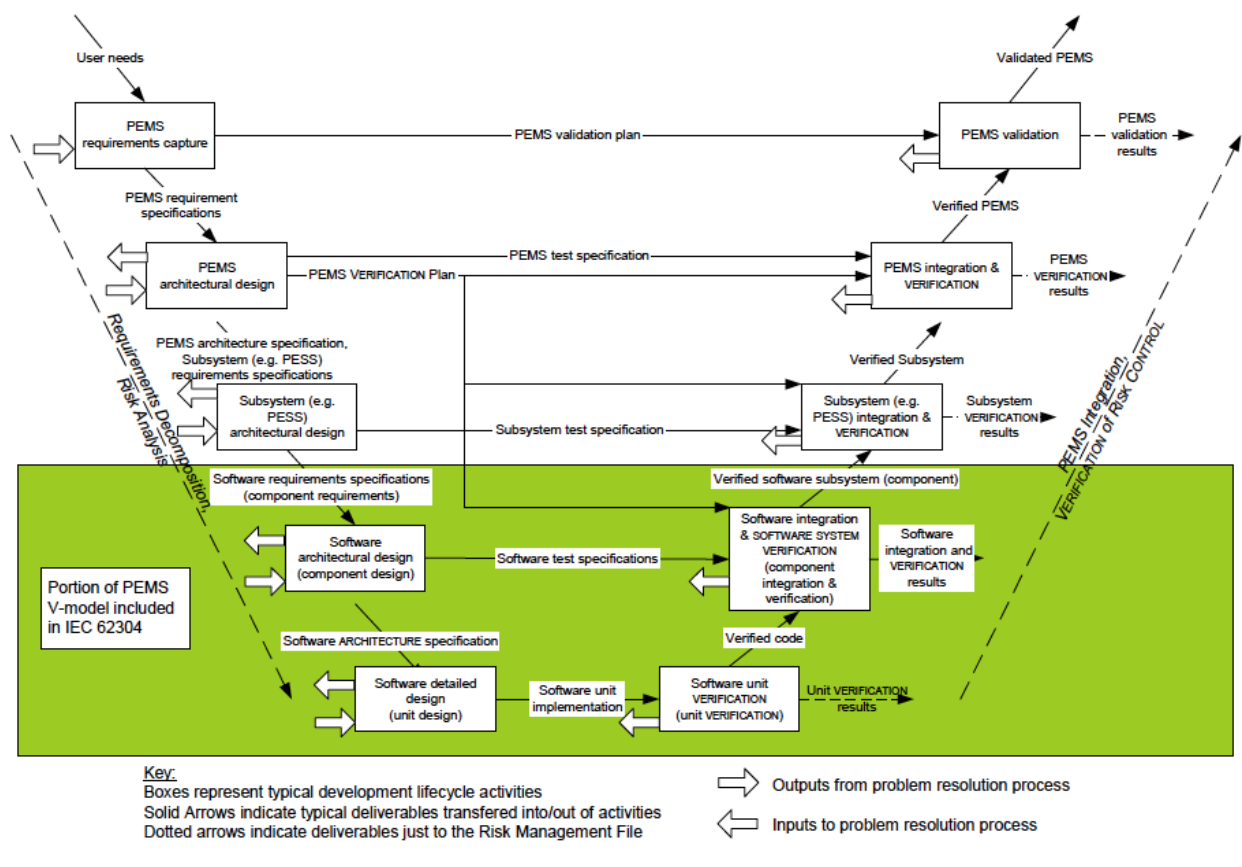
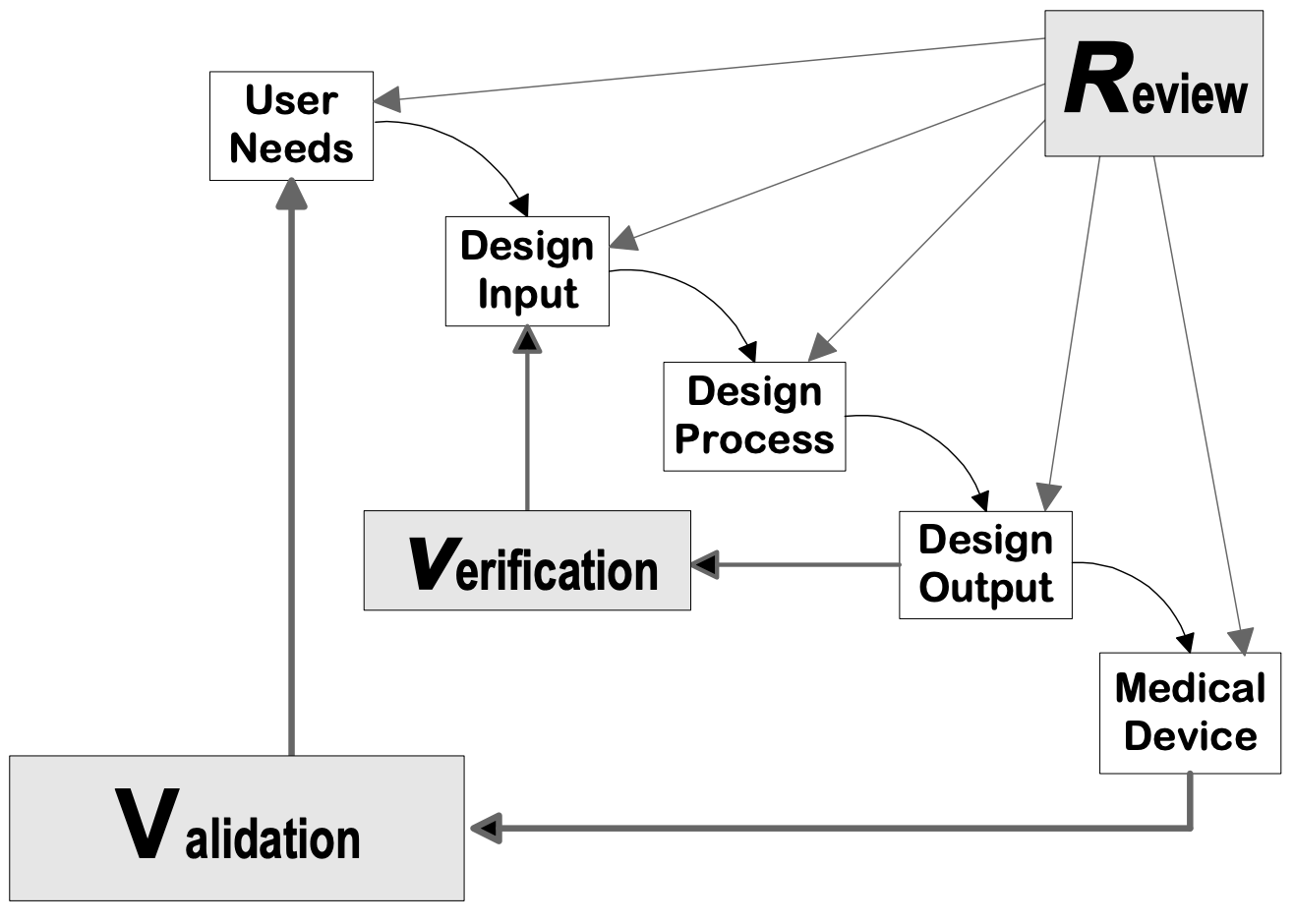
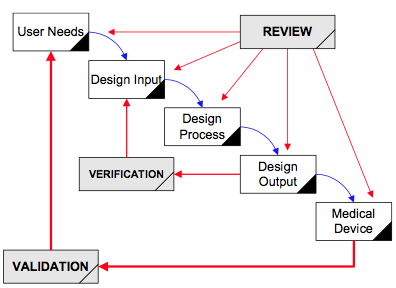
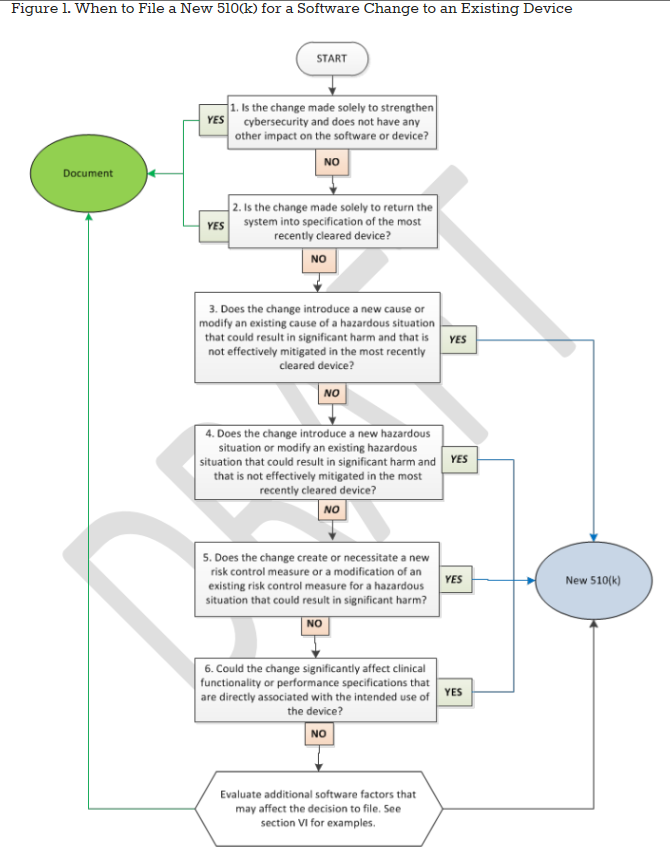
FDA Validation requirements for medical devices | Risk management, Change control, Statistical process control
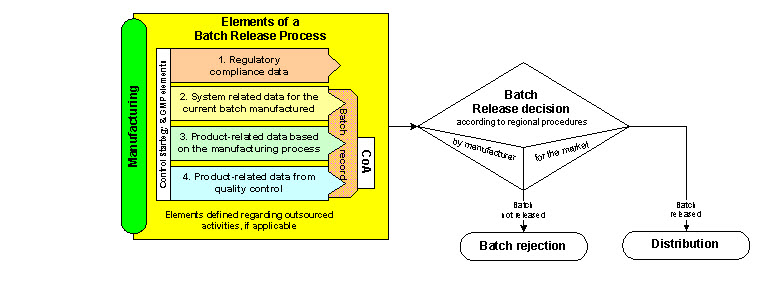
Q8, Q9, & Q10 Questions and Answers -- Appendix: Q&As from Training Sessions (Q8, Q9, & Q10 Points to Consider) | FDA
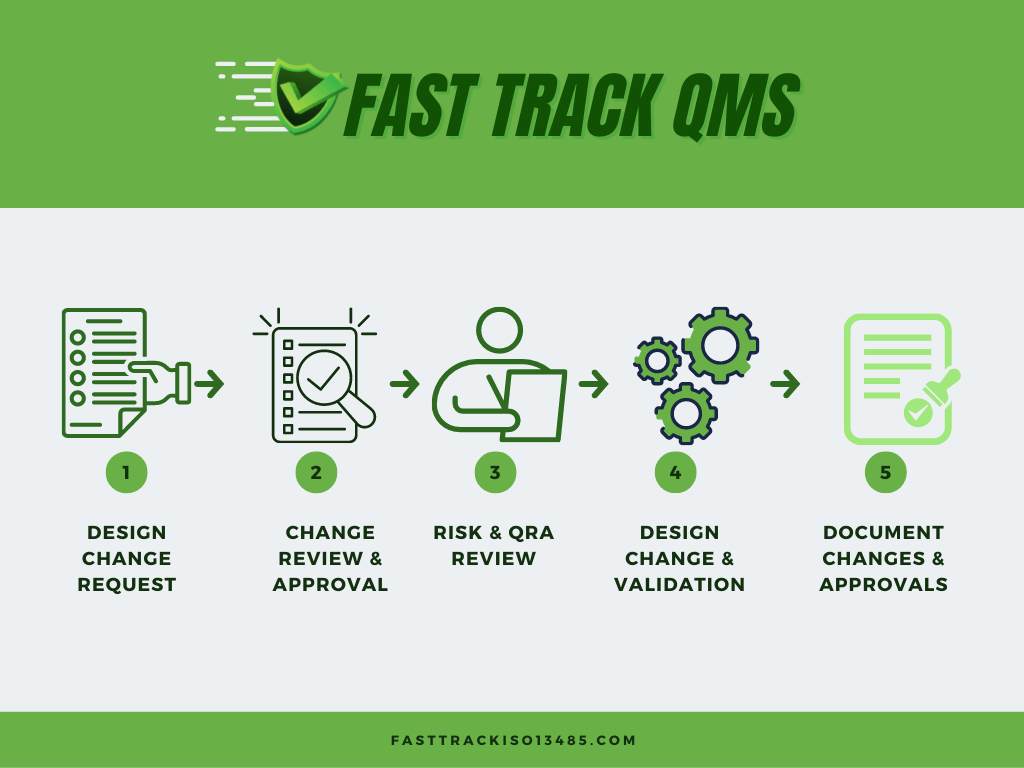
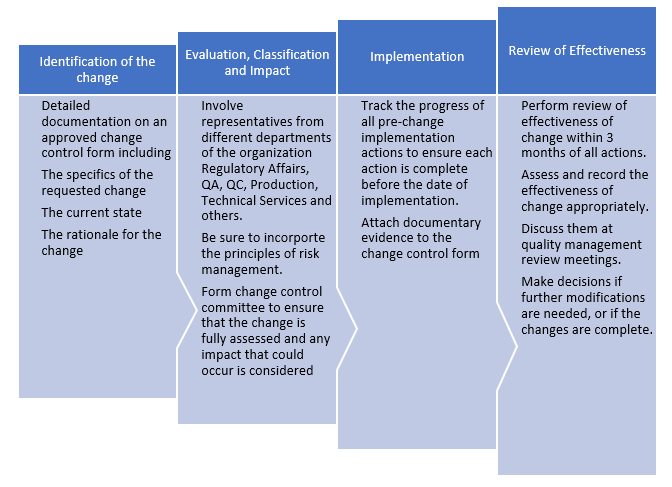
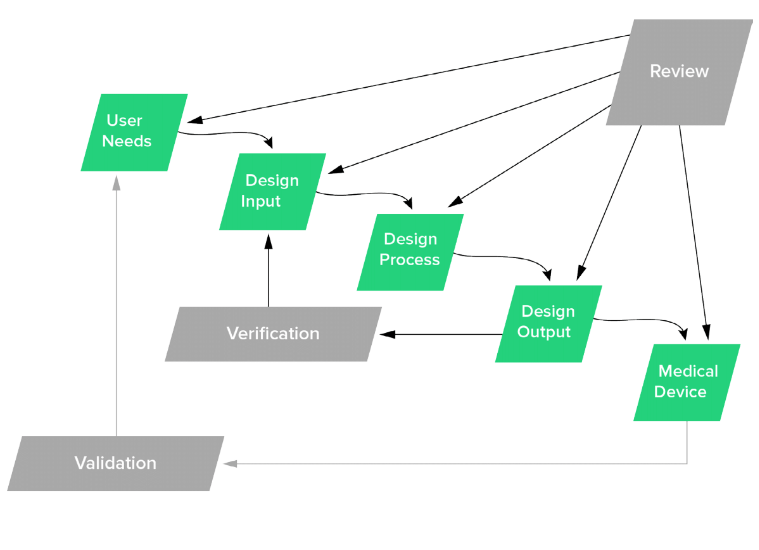
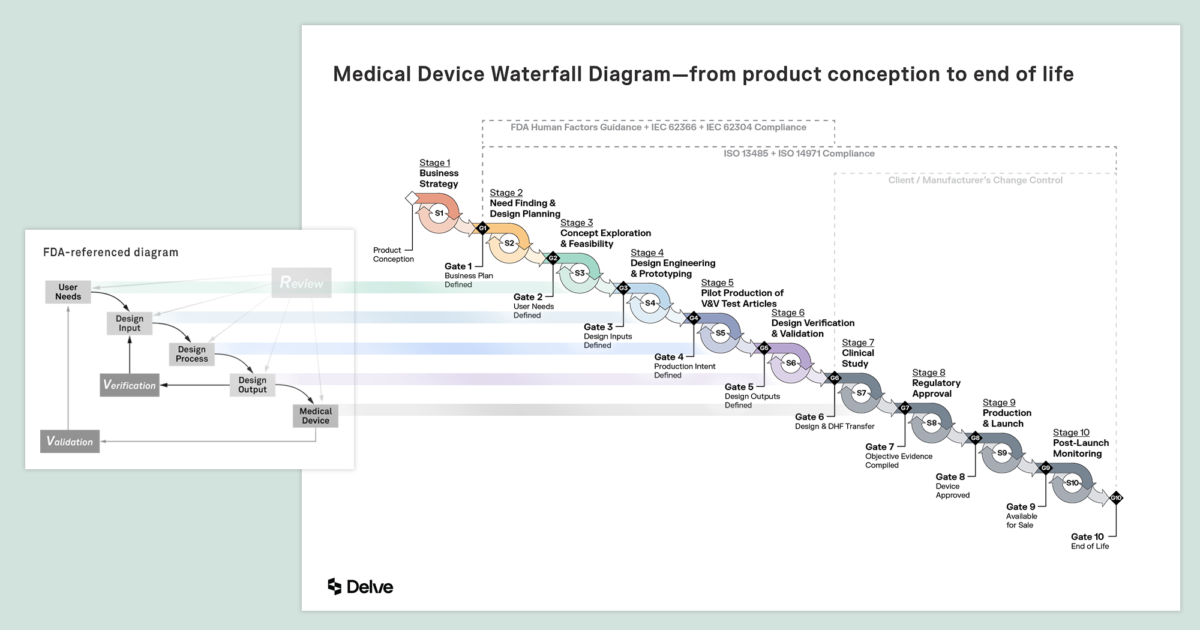
Fast Track ISO 13485 | How to Control Design Changes for your Medical Device and meet ISO 13485 requirements